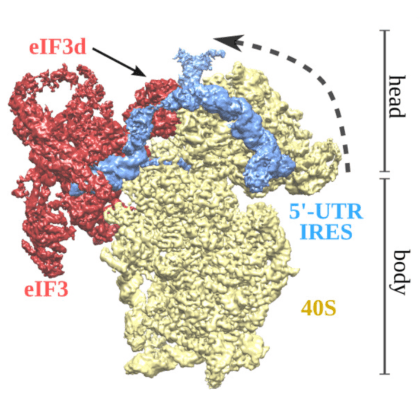
Instituto Biofisika (IBF) pursues a wide range of experimental and theoretical research themes under the umbrella term of Biophysics.

Instituto Biofisika (IBF) pursues a wide range of experimental and theoretical research themes under the umbrella term of Biophysics. Scientists with diverse backgrounds - chemists, biochemists, biologists, physicists, mathematicians and engineers – work together to carry out cutting-edge fundamental and translational research in biophysics, with the ultimate goal of enhancing our mechanistic understanding of biological processes and the pathogenesis of disease. We aspire to be a leading scientific institution of international repute, renowned for its transformative research and innovative education.